Why are Li-ion energy cells made with more capacity but can only deliver limited discharging current below 2C rate? Or, why are Li-ion power cells made with higher discharging current (5C ~ 15C rate) but can only be available with less capacity?
The above two questions have induced a lot of curiosity for many people but the puzzles are still there nowadays and you can’t get a reasonable answer by Google searches. Only very few battery experts can answer these two questions for you. Let’s address these two questions today.
The answers can be obtained if you know how to dissect the IR (Internal Resistance) of a rechargeable Li-ion battery. Understanding the IR mechanism of a rechargeable Li-ion battery will not only help you understand the proper battery valuation but also enable you to wisely choose an optimized Li-ion battery to assemble battery packs for your applications. You may spend the least amount of installation cost by exploiting the battery for its longest lifetime capacity. However, this is a complicate topic and is beyond the scope of this blog post. We will just dissect the negative electrode (a partial IR mechanism) of a rechargeable Li-ion battery and answer the above two questions. Please look at the following two drawing.
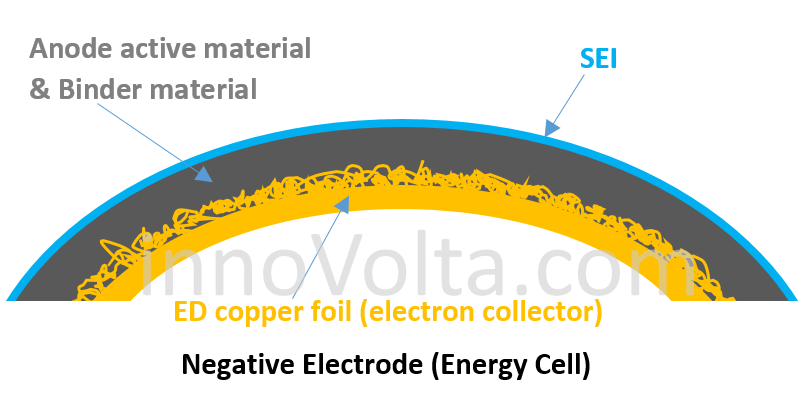
The electron collector (shown in gold color) on the negative electrode of an energy cell is made of ED (Electrodeposited) copper foil, which allows more space to accommodate more anode active material. Thus, it creates more capacity but comes with a much higher resistance (higher IR) to slow down electrons flowing through the binding interface, which limits energy cells to deliver a large current. If you abuse an energy cell over 2C rate, the battery body temperature will rise above 75 degree C easily. Keep on abusing it, the battery may run into thermal runaway if its body temperature is over 130 degree C!
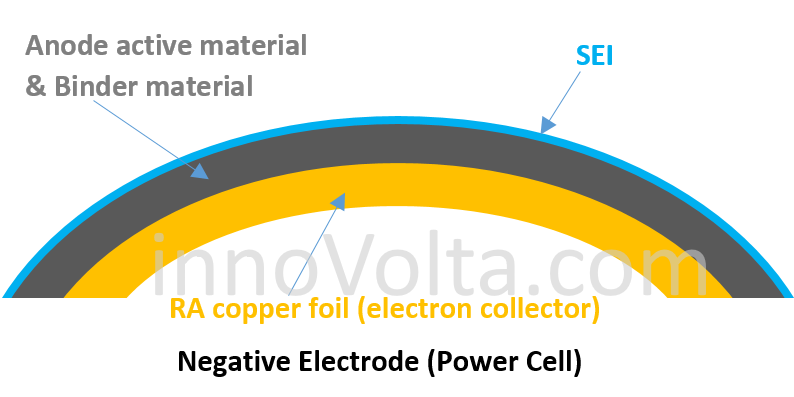
The electron collector on the negative electrode of a power cell is made of RA copper foil (Rolled copper Alloy), which reduces the space and can only accommodate less anode active material. Thus, it creates less capacity but comes with a much less resistance (lower IR) to speed up electrons flowing through the binding interface, which allows power cells to deliver a large current. If you let a power cell to drive an electrical load at its rated C rate, the battery body temperature will normally be below 70 degree C and you may continuously exploit the battery at its rated C rate for 150 ~ 2,000 cycles, depending on the cathode structure (i.e. approximately 2000 deep cycles for Li(N0.5M0.3C0.2)O2 cells or about 150 deep cycles for LiMn2O4 cells) and the type of proprietary electrolyte additives being used.
To this point, many people will still tell you that you can’t judge which kind of Li-ion battery is better because each of them has different advantage for their specific applications. This sounds correct but you will find that it is not true if you calculate and compare their “Lifetime Capacity”, which is the average capacity multiplied by their deep cycles.
